Starting Fresh – Why Clean Cotton Yields Great Results
Clean, fresh cotton. Most of us think of the pastel and white flocked woman dancing in a floral breeze singing about how much she loves the feel of cotton fabrics in her clothing. To get to this blissful reaction of cotton fiber fabrics, you have to start fresh. It’s difficult to have well dyed cotton without starting with a clean base.
What is cotton made of?
Let’s look first at what makes up a cotton fiber. There are three main parts to its fiber: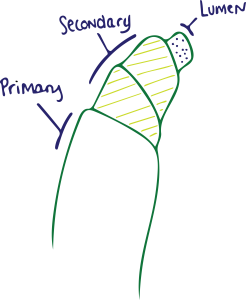
- Primary Wall (Outer layer)
- Secondary Wall (Middle layer)
- Lumen (Inner layer)
The Primary Wall consists of networks of cellulose fibrils, a small slender fiber or filament. The cuticle layer covers the wall of the fiber made of wax, pectin, protein, and mineral matter. It is the waxes that make the fiber waterproof and offer protection. It is our goal to remove these.
The Secondary Wall represents the majority of cellulose within the fiber. Most of the secondary wall is cellulose fibrils. The Lumen is the innermost part of the cotton fiber. Together, the raw cotton components then break down as follows:
- 80 – 90% Cellulose
- 68% Water
- 0.5 – 1% Waxes and Fats
- 0 – 1.5% Protein
- 4 – 6% Pectins
- 1 – 1.8% Ash
- 1 – 1.5% Other (Soluble Acids & Sugars)
Where do the impurities come from?
Impurities come from natural growth processes or outside factors like pesticide use. The most common parameters that affect the impurity levels in raw cotton are as follows:
- Regions where the cotton is grown
- Soil conditions including levels of calcium, magnesium, iron, and copper
- Weather conditions
- Growth, production, and harvesting chemicals (including fertilizers and pesticides)
- Insect secretions, oils, and greases from machinery
Why do I have to prep my fabrics?
Preparation of the fabric is critical to the end results in your process. Underprepared fabric can cause major problems in dyeing. The consistencies from lot to lot in production can vary. It can cause unlevel dyeings from port to port and side-center-side shading in dye lots. To combat this, you must guarantee bleaching uniformity. This process will remove impurities from the fabric and leave you with a clean slate. This success will determine the quality achieved at the end of the complete process.
Since cotton is a natural fiber, we need to scour or bleach the fabric to rid of the impurities. The earth’s soil, pesticides, and processing chemicals all influence the dyeing operation. Once removed from the cotton, the fibers have a uniform absorbency throughout the rolls. This allows even application of the dyes and finishing chemicals throughout the process. In taking these steps to prepare the cotton knit fabric, the dyeing and finishing process will be right the first time. The repeatability lot to lot will follow and the absorbency levels will be even throughout the fabric. It’s also possible to reduce the amount of finishing chemicals used, saving cost and process steps. Plus, you’ll have a softer hand in the finished garments (perfect for that commercial appeal).
How do I properly prepare cotton for dyeing?
Choosing the correct preparation procedures is important. It will make sure the cotton has a high and uniform absorbency throughout the process. The idea is to remove the waxes, oils, and some mineral contaminants found in the cotton. This process will improve quality and repeatability in production from lot to lot.
To start this process, you will need to determine if the fabrics need scouring or bleaching. Usually for dark and dull shades you can choose a scour. For bright and clean shades, you will need to do a bleach procedure. Then, determine the water level to use in the scour or bleach. This is important. You should use more water in a preparation stage to help remove impurities. If the liquor ratio is below 8:1, then the chemical additions should be in % rather than in g/l.
The preparation of the cotton fabric is the most critical step in your operation. The fabric should be well prepped to have absorbency throughout the rolls. The absorbency obtained in the preparation stage will also follow into finishing. This can reduce the amounts of chemicals you use to achieve your final specifications. This saves you time, energy, and money.
For best results, we recommend the following products and processes:
Application: Sora Scour LF-MD
Action:
Sora Scour LF-MD Conc is a multi-functional, low foaming detergent and wetting agent, APEO free, with emulsifying and extraction action for use in pretreatment of cellulose and cellulose blends with synthetic and/or spandex.
Jet or Package
Alkaline Boil Off:
Liquor Ratio: 1:8
Sora Scour LF-MD Conc: 0.5 – 1.5% owg
NaOH 50% 1.0 – 3.0% owg
Temperature 190 – 225° F
Time 30 – 45 minutes
Bleaching for Dyeing Cotton*:
Liquor Ratio: 1:8
Sora Scour LF- MD Conc 0.5 – 1.5% owg
Sora Stabilize CP 1.0% owg
NaOH 50% 1.0 – 3.0% owg
H2O2 50% 2.0 – 4.0% owg
Temperature 205 – 230°F
Time 30 – 60 minutes
Bleaching for Full White. Cotton*:
Liquor Ratio: 1:8
Sora Scour LF-MD Conc 0.5 – 1.5% owg
Sora Stabilizer CP 1.0% owg
NaOH 50% 2.0 – 4.0% owg
H2O2 50% 4.0 – 6.0% owg
Temperature 205 – 230° F
Time 30 – 45 minutes
*Note: When scouring or bleaching heavily soiled cotton or cotton containing high amounts of metals the addition of Soralon XT-PBS, 1.0% owg, will improve absorbency and degree of whiteness. Soralon XT-TBS also stabilizes the H2O2. The amount of the Sora Stabilizer CP can be reduced to 0.5%.
Looking to get started with a clean slate? Discover the best preparation formulation for you from our textile chemical portfolio.
One Comment
Comments are closed.



Great article Angelo. Comparing the needs or differences in processing between natural and synthetic fiber is very critical. My career has almost entirely evolved around synthetic processing and understanding this form of processing is very helpful.