Why You Need to Monitor Dye pH Sensitivity
Did you know that dyes can be sensitive to a change in pH? Most organic and natural dyes have this sensitivity. If ignored, it can result in colorations you weren’t expecting in your end result. It’s something to be aware of when selecting dyes. Let’s review exactly what pH sensitivity means so you can guarantee you’re working with products with compatible pH ranges for your processes.
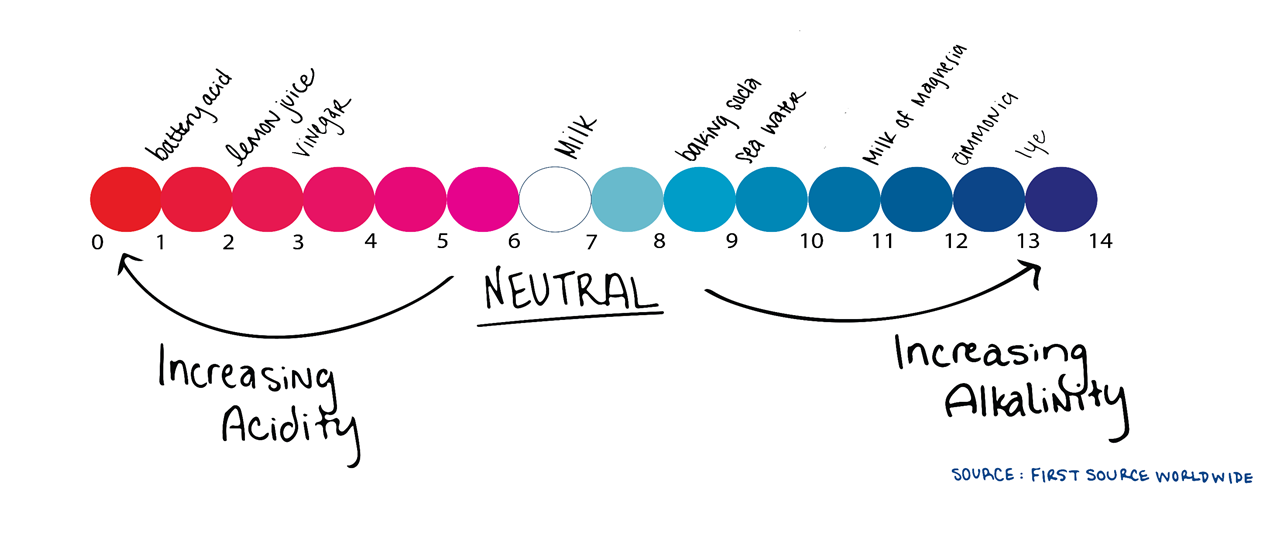
First we have to understand pH is a measure of how acidic or alkaline water is. The range goes from 0-14, with 7 being neutral. A pH of less than 7 indicates acidity, whereas a pH of greater than 7 indicates a base or alkalinity.

A simple example of something acidic is vinegar and something alkaline is Drano®. As stated above, water is neutral with a pH of 7.
Most dyes are not affected by a neutral pH, such as water. So what happens when we introduce a dye into an acidic or alkaline medium? There are three things that can occur:
- The color changes
- The color does not change
- The color is destroyed
These distinctions are important. Depending on the use of the dye, you may want one of those three things to happen, but definitely not the others.
Alkalinity and pH Sensitivity
If we introduce a certain dye to color a solution, we are hoping it does not change color from what’s expected. If the color altered, you might think something is wrong and your product is bad. Imagine if you are the manufacturer of Drano® and you pick a dye that is supposed to color Drano® blue. When you made it, it appeared blue, but two days later it yellowed. This would be bad because the blueness of Drano® is a trademark color. A changed shade can affect consistency, brand awareness, and may cause the customer to think the product is bad. In this case, the color change occurred because the dye had alkaline sensitivity, giving you the wrong result.
Acidity and pH Sensitivity
Now let’s look at a situation where a sock manufacturer wants to dye their socks red. In this process he needs an acid to fix (or dye) the color to the sock fabric. He adds his red dye solution to the socks in water, creating a nice red color. As soon as he adds the acid to finish his formulation – bingo – the socks turn blue. The dye must have a sensitivity to acid, causing the reaction to change the color to blue. Obviously, having blue instead of red socks is not a good thing. Having awareness of the pH sensitivity can keep you from inadvertently discoloring your products.
If we introduce a dye in each of the above examples and there is no color change, then the dyes are stable in those pH systems. Thus, they would not change color. This is your goal when selecting dyes to add to existing formulations.
In some cases the color can be completely destroyed if the pH is either very alkaline (pH of 13) or very acidic (pH of 1). What happens is that the dye color turns clear shortly after being introduced to those situations. In other words, the color is destroyed. This is helpful for water treatment processes, but probably not for dyeing something like socks or a household cleaner.
The pH is an important factor when considering what dye you want to use. Ensure you know what the pH is of the solution the dyes will be dissolved in. Also consider if there is going to be anything added that might cause a change in pH of the original solution. Choose your dyes with caution.
Are you having pH problems with your dye processes? FSW’s team of technical staff can help you identify the areas affecting your results and provide trusted solutions.
One Comment
Comments are closed.



Hi Jim, Great blog. I know some tanneries that are having intermittent problems with a black dye that changes shade when fixing with formic. Maybe you could help!